Home Industry Biotech Biotech Firm Contraline Reache...
Biotech
CIO Bulletin
30 April, 2025
ADAM from Contraline Biotech proved successful as a safe male contraceptive according to the results of its clinical trials spanning 24 months.
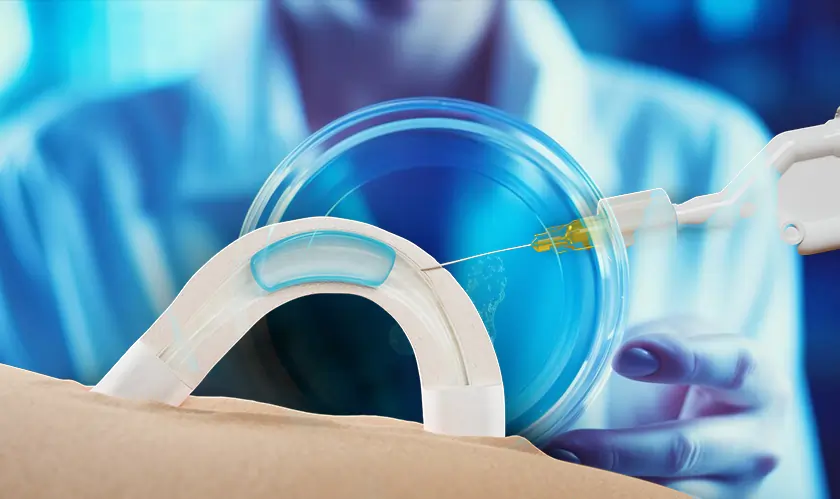
ADAM received a significant advancement in clinical tests from Contraline Biotech when the company reported its non-hormonal male contraceptive's positive results. The first stage of clinical experiments indicates that ADAM demonstrates both safety and effectiveness with 24 months of assessment. The complete clinical data will be released sometime after Contraline delivers more findings during the American Urological Association meeting on April 26.
The male contraceptive technology ADAM applies a water-soluble hydrogel to vasa deferentia where it prevents sperm while enabling normal ejaculation. The Biotech innovation provides a definite reversible technique for birth control that acts as an alternative to vasectomies and condoms for male contraception.
The Biotech solution from Contraline functions as an extended birth control option to address consumer preferences according to Alexander Pastuszak who serves as the chief medical officer at Contraline. The sperm counts in early trial subjects reached zero absolute at month 24 without any severe reported health complications.
Some experts want additional reversal information before supporting this development although Contraline makes strides that suggest a future transformation of reproductive health. The Biotech firm received regulatory approval for phase two test evaluations that will assess both safety and effectiveness of their product.
The successful implementation of ADAM would provide men an extended and reliable prevention method similar to female IUDs thereby revolutionizing the landscape of family planning.







