September Monthly Edition 2025
CIO Bulletin

Cancer remains one of humanity’s greatest challenges, often striking silently and evading detection until treatment becomes complex and uncertain. Millions worldwide face the fear and uncertainty of delayed diagnoses, limited treatment options, and the looming threat of recurrence. The need for early, accurate, and accessible cancer screening has never been more urgent. Creatv Bio, a division of Creatv MicroTech, Inc., offers transformative solutions, empowering patients and doctors with tools to detect and combat cancer when it matters most.
Creatv Bio leads with its LifeTracDx® blood tests, designed to detect cancer at its earliest, most treatable stages. Using innovative CellSieve™ microfilters, these tests isolate and identify critical cancer-associated cells, including the unique Cancer-Associated Macrophage-Like (CAML) biomarker, delivering precise insights to guide tailored treatments, monitor progress, and predict outcomes. From early screening to detecting recurrence, LifeTracDx® serves as a lifeline across diverse cancer types.Fueled by a mission to transform cancer care, Creatv Bio combines scientific excellence with genuine compassion, creating a world where early detection saves more lives and restores hope.
At CIO Bulletin, we were honored to interview Dr. Cha-Mei Tang, Sc.D., President and CEO of Creatv Bio. She shared valuable insights on how her company, by harnessing cutting-edge technology and a deep commitment to improving lives, is redefining the global fight against cancer—bringing clarity, confidence, and hope to people worldwide.
From Precision Engineering to Pioneering Oncology: The Origin of Creatv Bio
Sometimes, the most groundbreaking journeys begin with a detour. For Dr. Cha-Mei Tang, CEO of Creatv Bio, what started as a microfabrication venture became an unexpected force in cancer diagnostics—driven by discovery rather than design.
“Creatv MicroTech was incorporated in Delaware in 2000, focusing on microfabrication and the detection of pathogens. Switching the company’s focus to cancer diagnostics and cancer detection was unplanned, the result of two events,” says Dr. Cha-Mei Tang.
Initially, the company specialized in high-precision microfabrication. Then came a unique request: to develop a microfilter to isolate Circulating Tumor Cells (CTCs) from blood samples.
As Dr. Tang explains, “Creatv MicroTech provided microfabrication services. We were asked to make a microfilter to collect Circulating Tumor Cells (CTCs) (Figure 1) from the blood of cancer patients. We did an exceptional job making the filter, testing the filter, and comparing it to other commercially available filters. This took a while. By the time we contacted our customer, they had already decided to use another supplier. So, we decided to commercialize it ourselves, calling it the CellSieve™ microfilter.”
This decision would lay the groundwork for a discovery that would redefine the company’s future. While testing the CellSieve™ system, the team observed something no one had documented before.
“Our blood test stood out for another unexpected reason. There are many technologies on the market for collecting CTCs. For example, the CellSearch instrument, part of the Johnson & Johnson company in 2014, received three FDA approvals for capturing circulating tumor cells (CTCs) in breast, prostate, and colorectal cancer patients’ blood. We developed CTC detection assays using cancer cell lines before we processed cancer patient samples. But in the first cancer patient sample we processed, we found a type of very large cell, but there were no research publications on them. We had to validate that others had seen them, but no one had studied them. To verify that we had not found junk in the blood, we reached out to the CellSearch people. They told us they had seen those giant cells, but those cells are not CTCs. They did not study them.”
That anomaly caught the attention of Chief Scientific Officer Daniel L. Adams, who made a groundbreaking observation.
“Daniel L. Adams, our Chief Scientific Officer, identified them as macrophages that had engulfed tumor cells. We named these cells Cancer Associated Macrophage-like Cells (CAMLs) (Figure 2). This began our unexpected journey with the LifeTracDx® blood test.”
That journey led to the formation of a new division—one fully focused on advancing cancer diagnostics.
“Creatv Bio, a division of Creatv MicroTech, was formed after significant support for clinical applications of the LifeTracDx® blood test,” states Dr. Cha-Mei Tang.
What began as a precision engineering project evolved into a clinical innovation with the potential to change how cancer is detected. For Dr. Tang and her team, the path wasn’tplanned—but it was pioneering.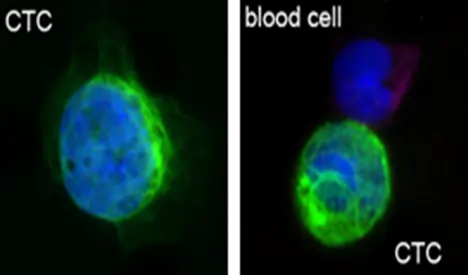
Figure 1. Images of circulating tumor cells (CTCs). The dark blue represents the fluorescent image of the nucleus. The green filaments are cytokeratin conjugated with green fluorescent dyes. In live CTCs, cytokeratins appear in the form of filaments.
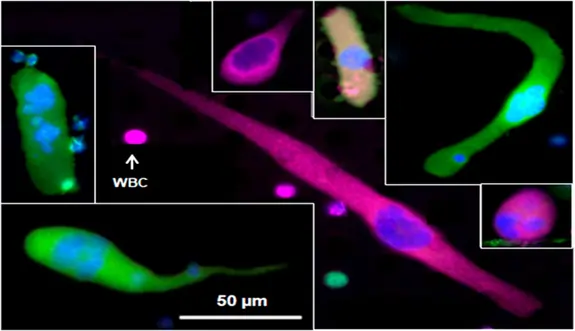
Figure 2. Images of cancer-associated macrophage-like cells (CAMLs). The white arrow points to a white blood cell (WBC). CAMLs are typically 25–300 microns in size. They are polynucleated (dark blue) as a result of the tumor cells they have engulfed. When small, they are round or oval-shaped. Sometimes, they appear as large rod-shaped cells. Often, they have one tail or two tails on opposite sides of the cell. The tails can be very long. CAML cells are much larger than CTCs.
LifeTracDx®: Revolutionizing Cancer Diagnostics with Speed, Accuracy, and Personalization
At Creatv Bio, innovation extends beyond the lab, transforming how clinicians detect, monitor, and understand cancer. At the heart of this transformation is LifeTracDx®, a cutting-edge liquid biopsy that captures and identifies Cancer-Associated Macrophage-Like Cells (CAMLs)—a groundbreaking assay with far-reaching implications. As Dr. Tang explains, the test’s versatility and impact are life-changing.
A Powerful Diagnostic Tool with Broad Applications
The LifeTracDx® blood test’s most remarkable feature is its versatility. “The test has broad applications because CAMLs are found in over 30 different types of cancer. Most importantly, they appear at all stages, making this test suitable for both diagnostics and cancer screening.”
Predicting Treatment Response Early
One standout feature of LifeTracDx® is its ability to predict treatment response within 30 days. “If the number of CTCs or CAMLs increases, or if CAML size increases, it indicates the patient is not responding to therapy. This allows patients to switch therapies sooner, without waiting for imaging to show tumor growth, which can significantly improve survival and quality of life.”
Companion Diagnostics for Targeted Therapies
LifeTracDx® also can be developed to serve as a companion diagnostic for various therapies. “These blood tests don’t require tissue samples and can determine if a patient’s tumor has the right marker for a specific drug. CAMLs, which are macrophages engulfing tumor cells, carry all tumor markers, making them ideal for developing companion diagnostics.”
For patients undergoing immunotherapy, determining whether tumor cells express the PD-L1 marker is critical. “Immunotherapy enables T-cells to kill tumor cells, but its effectiveness depends on PD-L1 presence. LifeTracDx® can assess PD-L1 expression by analyzing CTCs and CAMLs, helping doctors determine if a patient will benefit from immunotherapy,” she adds.
Monitoring Cancer Aggression and Treatment Progress
LifeTracDx® provides vital information throughout the treatment journey. “We’ve identified 10 features on CTCs and CAMLs that indicate cancer aggressiveness. This allows physicians to assess how aggressive a patient’s cancer is and whether the current treatment plan remains appropriate.”
It also tracks changes in tumor biomarkers. For example, a drug’s target may disappear, or PD-L1 expression may fluctuate, indicating potential benefits from immunotherapy. “We have data showing LifeTracDx® can detect these changes, ensuring patients receive the right therapy at the right time.”
Detecting Minimal Residual Disease and Cancer Recurrence
The test plays a pivotal role post-treatment. “If CAMLs are still found in the blood after therapy ends, it signals that the cancer hasn’t been fully eradicated.” This detection of minimal residual disease (MRD) prevents false reassurance from the absence of visible tumors.
LifeTracDx® also detects early cancer recurrence, which is crucial. “Cancer often returns even after successful therapy. Early detection is key to effective re-treatment and better outcomes,” Dr. Tang emphasizes.
Providing Whole Tumor DNA for Accurate Sequencing
LifeTracDx® offers a unique feature: providing whole tumor DNA for sequencing. “Whole tumor DNA provides more accurate results than short fragments of circulating tumor DNA (ctDNA) in the blood. This helps identify mutations and therapeutic targets that might otherwise be missed.”
While Creatv Bio can detect cancer early for screening, Dr. Tang remains focused on diagnostics and treatment monitoring. “Our current focus is on supporting patients during their treatment journey, ensuring early detection of changes and the most accurate data possible.”
CAMLs: A Groundbreaking Discovery Reshaping Cancer Detection and Treatment
Dr. Cha-Mei Tang reflects on the journey that led to the discovery of Cancer-Associated Macrophage-Like cells (CAMLs), now central to LifeTracDx®. This innovation is transforming cancer detection and monitoring, offering patients better chances for early intervention and improved outcomes.
“We collaborated with oncologists over 12 years, across various cancer types and therapies,” says Dr. Tang, acknowledging their crucial role. In 2014, Creatv Bio published its initial findings on CAMLs. Other researchers have since explored these cells, often under different names. “The NIH calls them Tumor Macrophage Hybrid Cells (TMHCs). In February 2024, MD Anderson Cancer Center hosted the first international conference on these cells.”
CAMLs are a game-changer due to their presence across multiple cancer types, both solid and liquid, and at all stages. “We’ve detected CAMLs in every cancer type we’ve studied.” This suggests the potential capability of early detection and better treatment monitoring.
Creatv Bio’s LifeTracDx® assay captures various cancer-associated cells, including CTCs, EMT CTCs, TMHCs, and CAMLs. However, Dr. Tang stresses the importance of distinguishing between these cells. “Many researchers fail to do so due to technological limitations, which can harm patients. Some companies incorrectly label all cancer cells as CTCs, which isn’t accurate.”
By precisely identifying cell types, LifeTracDx® enhances cancer screening, monitoring, and recurrence detection, leading to more accurate diagnoses and better patient care. Through CAMLs, Creatv Bio is revolutionizing cancer diagnostics with a precise, comprehensive approach.
Transforming Cancer Treatment with Real-Time Precision
Creatv Bio is revolutionizing cancer care with LifeTracDx®, offering real-time insights into tumor mutations, drug target expression, and treatment response within 30 days. “LifeTracDx can help many cancer patients survive longer, benefiting a wide range of cancer types.”
The test identifies how cancer evades the immune system. “Cancer cells cover their surface with PD-L1 markers, preventing T-cells from attacking.” Immunotherapy drugs block PD-L1 to enable T-cells to fight cancer, but they only work if PD-L1 is expressed. Unlike traditional PD-L1 tests requiring tissue samples, LifeTracDx® uses a blood test to assess PD-L1 during therapy.
LifeTracDx® monitors PD-L1 expression in real-time, even as it changes during treatment. In a lung cancer clinical trial, chemo-radiation therapy shifted patients’ PD-L1 levels, improving immunotherapy outcomes. “This showed the importance of monitoring PD-L1 during therapy.”
In a Phase I FDA trial for metastatic breast cancer, LifeTracDx® revealed that two-thirds of patients with low PD-L1 developed higher levels after treatment. “Patients with medium or high PD-L1 survived up to four years,” she adds.
In a renal cell carcinoma case, LifeTracDx® identified a biomarker shift, prompting a therapy change. “The patient’s cancer stabilized after switching to immunotherapy.”
“PD-L1 expression can change during therapy, and continuous monitoring is crucial. LifeTracDx® is the only test with this capability.”
Creatv Bio’s innovation enables real-time, personalized cancer treatment, empowering patients and doctors to make informed decisions at every stage.
A Look into the Future
Creatv Bio’s mission is clear: to support cancer patients by helping them choose the best therapy and track its effectiveness. “We are not a drug company, but we can help patients select the best therapy and confirm it’s working,” says Dr. Tang.
The company’s long-term goals are ambitious. “Our goal is to obtain FDA approvals for all our diagnostics, covering cancer screening and diagnostics for all cancers, stages, and therapies. This will be a long and costly process, but we are committed.” This requires significant investment and collaboration. “We will need support from venture capitalists, collaborators, and partners.”
Creatv Bio is currently in the research mode. In the near future, it is Creatv Bio’s goal to establishing a CLIA laboratory and preparing FDA applications, marking a significant milestone. “This is just the beginning. We are poised to make real-time, personalized cancer treatment a reality for all,” concludes Dr. Cha-Mei Tang.
Creatv Bio | Leadership
Dr. Cha-Mei Tang, Sc.D., is the Founder, President, and CEO of Creatv MicroTech. She earned her Bachelor’s, Master’s, and Doctoral degrees from the Department of Electrical Engineering and Computer Science at the Massachusetts Institute of Technology. After graduation, she conducted theoretical physics research on relativistic electron beams and free electron lasers at the Naval Research Laboratory. Dr. Tang has diverse interests—she even considered a doctoral thesis on acupuncture. When the opportunity arose to leave the Naval Research Laboratory, she founded Creatv MicroTech without hesitation.
Daniel L. Adams, the Chief Scientific Officer, is a key figure in the company. He developed the assays, analyzed data, and identified their clinical applications.

Insurance and capital markets







